Identifying outlier utilization of Synagis during the RSV season
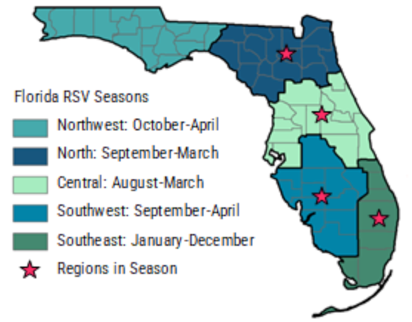
Respiratory syncytial virus [RSV] is a common infection that usually produces mild, cold-like symptoms. Patients diagnosed with RSV and who have pre-existing respiratory issues or immune-compromised systems, such as pediatric patients born prematurely, may become infected with pneumonia, bronchiolitis, and other respiratory tract issues that result in hospitalization. The RSV season for nearly every region of the United States spans five months typically starting in November and concluding in March. Of interest, the onset and conclusion of the RSV season in Florida varies from other regions across the United States, and also has distinct sub-regional patterns across the state.RSV-NET Participating States
Florida RSV season and sub-regions
Public Health Intelligence
Codoxo collected and analyzed the population data from the Centers for Disease Control (CDC), Emerging Infections Program, and RSV-NET [Respiratory Syncytial Virus Hospitalization Surveillance Network], which is a system that collects weekly reporting of laboratory-confirmed RSV-associated hospitalizations from 12 participating states and 58 counties. The RSV-NET system documents hospitalization rates per 100,000 people, covers approximately 8-9% of the population in the United States, and provides the number and percentage of affected populations in non-reporting areas which could vary from the overall population. During the 2018 RSV season, the CDC updated their tracking of RSV hospitalizations across all age brackets compared with the prior RSV seasons that only included data for the adult population [18 years and older]. Unfortunately, RSV-NET reporting during the 2020 season was very limited due to the COVID-19 pandemic and as such, Codoxo omitted those limited values from the collection and analysis.
The following chart represents the cumulative laboratory-confirmed RSV-associated hospitalizations during the first six weeks of the 2018, 2019, 2021 and 2022 RSV seasons for the highest-risk populations.
Overall count of RSV hospitalizations per 100,000 people [Source CDC RSV-NET]
|
Age Group RSV Season |
0-<6 months | 6-<12 months | 1-<2 years | 2-4 years | 5-11 years |
| 2018-2019 | 75.1 | 33.7 | 23.8 | 6.2 | 0.9 |
| 2019-2020 | 99.5 | 52.4 | 25.2 | 10.1 | 0.7 |
| 2021-2022 | 328.8 | 94.2 | 72.3 | 24.4 | 3.1 |
| 2022-2023 | 817 | 405.7 | 234.2 | 115.8 | 15.1 |
The following chart identifies the increase of hospitalizations by percentage between the first six weeks of the prior RSV seasons [2018, 2019, 2021] to the first six weeks of the 2022 season for the high-risk populations.
Percentage of increase of RSV hospitalizations [Source Codoxo]
|
Age Group RSV Season |
0-<6 months | 6-<12 months | 1-<2 years | 2-4 years | 5-11 years |
| 2018-2019 | 1088% | 1204% | 984% | 1868% | 1678% |
| 2019-2020 | 821% | 774% | 929% | 1147% | 2157% |
| 2021-2022 | 248% | 431% | 324% | 475% | 487% |
Synagis treatment, dosing, and administration
Synagis [palivizumab] is an FDA-approved, monoclonal antibody, injectable [intramuscular] treatment to prevent acute lower respiratory infection [ALRI] for pediatric patients that have a high-risk of RSV infection based on diagnosed conditions:
-
- History of premature birth (less than or equal to 35 weeks gestational age) and who are 6 months of age or younger at the beginning of RSV season
- Bronchopulmonary dysplasia (BPD) that required medical treatment within the previous 6 months and who are 24 months of age or younger at the beginning of RSV season
- Hemodynamically significant congenital heart disease (CHD) and who are 24 months of age or younger at the beginning of RSV season
The following table of diagnosed conditions and ICD-10 CM codes/ranges are associated with high-risk patient populations with a confirmed RSV diagnosis.
Diagnosed conditions and ICD-10 CM codes
| Diagnosed Condition | ICD-10 CM Codes or Range |
| Respiratory syncytial virus | B974 |
| Cardiomyopathy, unspecified | I429 |
| Heart failure, unspecified | I509 |
| Premature birth | P0721 to P0738 |
| Bronchopulmonary dysplasia | P271 to P279 |
| Pulmonary hypertension of newborn | P2930 |
| Arterial/Ventricle/Coronary/Aortic/Venous [range of issues] | Q200 to Q269 |
Your health plan may offer coverage for diagnosed conditions in addition to the FDA recommendations. Please review and consider those conditions when reviewing preauthorization requests for Synagis.
The treatment frequency for Synagis is once per month during the RSV season. Synagis treatment should start at the onset of the RSV season and typically results in a maximum of 5 treatments. One notable exception to the frequency of Synagis administration is for pediatric patients undergoing cardio-pulmonary bypass. Since Synagis serum levels decrease after a cardio-pulmonary bypass, those pediatric patients should receive an additional dose of Synagis as soon as possible after the cardio-pulmonary bypass procedure (even if sooner than a month from the previous dose). Thereafter, doses should be administered monthly as scheduled.
Synagis is distributed in two dosage types, 50mg per 0.5mL and 100mg per 1mL. Synagis does not contain preservatives and the vials are single-dose solutions, which means the provider cannot re-use the vial for another patient. Synagis dosages are based on the weight of the patient. The dose (volume of injection in mL) per month = patient weight (kg) x 15 mg per kg ÷ 100 mg per mL of Synagis. Injection volumes over 1 mL should be given as a divided dose.
Preauthorization and Administration
Synagis is an expensive, prophylactic treatment that requires preauthorization, and many plans allocate its distribution and management to a single specialty pharmacy or as needed, a home health agency. Individual providers may provide the injection service; however, due to distribution controls, it is unlikely an individual provider would receive payment for the drug itself. Synagis claims may appear in a health plan as a medical benefit or pharmacy benefit, identified by a CPT, HCPCS or NDC code. Synagis injections can occur in multiple settings, most commonly the physician’s office, the patient’s home, or a facility.
Specialty pharmacies in collaboration with health plans have Synagis pre-authorization forms that are submitted by the prescribing physician. The prescribing physician requests the number of doses/milliliters for Synagis and outlines the pediatric patient’s supporting medical history, which should include the patient’s diagnosed conditions and most importantly, the patient’s weight. The health plan staff, likely the Medical Director, will review and approve the request from the prescribing physician and then the specialty pharmacy will supply the approved dosages.
CPT, HCPCS, and NDC codes associated with Synagis and drug administration [Current for Q4-2022]
| Codes | Description |
| 90378 | Respiratory syncytial virus, monoclonal antibody, recombinant, for intramuscular use, 50 mg, each |
| 96372 | Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); subcutaneous or intramuscular |
| S9562 | Home injectable therapy, palivizumab, including administrative services, professional pharmacy services, care coordination, and all necessary supplies and equipment (drugs and nursing visits coded separately), per diem |
| 66658-230-01 | Synagis [palivizumab], 50-mg vial |
| 66658-0230-01 | Synagis [palivizumab], 50-mg vial |
| 66658-231-01 | Synagis [palivizumab], 100-mg vial |
| 66658-0231-01 | Synagis [palivizumab], 100-mg vial |
Codoxo ‘weighs in’ on patient weight
Due to the single-use vials with different dosages and the dependencies on weight calculations to determine the appropriate dosage, one concern with Synagis is excessive dosage. As mentioned above, the high-risk population are pediatric patients with additional medical conditions, such a premature birth, which may affect their weight compared to a patient born at full-term. According to the FDA guidelines, the efficacy of Synagis administration in pediatric patients older than 24-months at the start of dosing has not been established.
Codoxo collected age-to-weight growth charts from the CDC for pediatric patients aged 0-24 months, reviewed studies related to premature births and compensatory growth/gains, and identified the ICD-10 CM codes specific to gestation periods, birth weight, and pediatric patient benchmarks by percentile.
Premature births are defined as a newborn delivered at less than 37 weeks of gestation or a newborn whose weight is under 2500 grams. According to the CDC, the annual rate for premature births is around 10% of the total newborn population. Approximately 8-9% of the total newborn population weigh between 2500 and 1501 grams, and the remaining population are under 1500 grams.
Diagnosis codes [Low birth weights, gestation periods, pediatric benchmark by percentile]
| ICD-10 CM | Description |
| P0700 | Extremely low birth weight newborn, unspecified weight |
| P0701 | Extremely low birth weight newborn, less than 500 grams |
| P0702 | Extremely low birth weight newborn, 500-749 grams |
| P0703 | Extremely low birth weight newborn, 750-999 grams |
| P0710 | Other low birth weight newborn, unspecified weight |
| P0714 | Other low birth weight newborn, 1000-1249 grams |
| P0715 | Other low birth weight newborn, 1250-1499 grams |
| P0716 | Other low birth weight newborn, 1500-1749 grams |
| P0717 | Other low birth weight newborn, 1750-1999 grams |
| P0718 | Other low birth weight newborn, 2000-2499 grams |
| P0720 | Extreme immaturity of newborn, unspecified weeks of gestation |
| P0721 | Extreme immaturity of newborn, gestational age less than 23 completed weeks |
| P0722 | Extreme immaturity of newborn, gestational age 23 completed weeks |
| P0723 | Extreme immaturity of newborn, gestational age 24 completed weeks |
| P0724 | Extreme immaturity of newborn, gestational age 25 completed weeks |
| P0725 | Extreme immaturity of newborn, gestational age 26 completed weeks |
| P0726 | Extreme immaturity of newborn, gestational age 27 completed weeks |
| P0730 | Preterm newborn, unspecified weeks of gestation |
| P0731 | Preterm newborn, gestational age 28 completed weeks |
| P0732 | Preterm newborn, gestational age 29 completed weeks |
| P0733 | Preterm newborn, gestational age 30 completed weeks |
| P0734 | Preterm newborn, gestational age 31 completed weeks |
| P0735 | Preterm newborn, gestational age 32 completed weeks |
| P0736 | Preterm newborn, gestational age 33 completed weeks |
| P0737 | Preterm newborn, gestational age 34 completed weeks |
| P0738 | Preterm newborn, gestational age 35 completed weeks |
| P0739 | Preterm newborn, gestational age 36 completed weeks |
| Z6851 | Body mass index [BMI] pediatric, less than 5th percentile for age |
The weight of a pediatric patient is the essential piece of information for calculating the Synagis dosage. Premature newborns or patients under two years old that were born prematurely or with a documented low birth weight or low population percentile, will have a lower weight than a patient born at >37 weeks of gestation or full-term. Another important consideration of premature pediatric patients is compensatory growth or gains compared to the other 90% of the newborn population. Head circumference and body length are areas of growth that ‘catch-up’ to the 90% within two to three years; however, weight gain does not follow the same trend.
Codoxo calculated the monthly growth ratio for male and female pediatric patients based on the CDC growth charts. As expected, the growth rates were higher in the early months and the growth rate reduces as they progress. The average monthly growth ratio for males is 1.055 and the average monthly growth ratio for females is 1.054. In consideration of compensatory growth or gains for premature patients, we applied a growth ratio of 1.0725 to birth weights within the premature weight ranges to account for part of the premature population reaching a percentile commensurate with the full-term birth population.
While the dosage amounts based on patient weight for Synagis are constant regardless of the patient age, health plans should consider the patient history, specifically those patients born prematurely. For example, the number of vials [50ml] for a full-term pediatric patient in the 75th percentile between the age of 10 months and 21 months would be three to four vials [males=4, females=3]. Conversely, the dosage for a pediatric patient, whose premature birth weight was in the 75th percentile [above 1500 grams and below 2500 grams] would be two to three vials. Below are the 75th percentile weights [birth to 24 months] and dosage calculations for Synagis across the three populations.
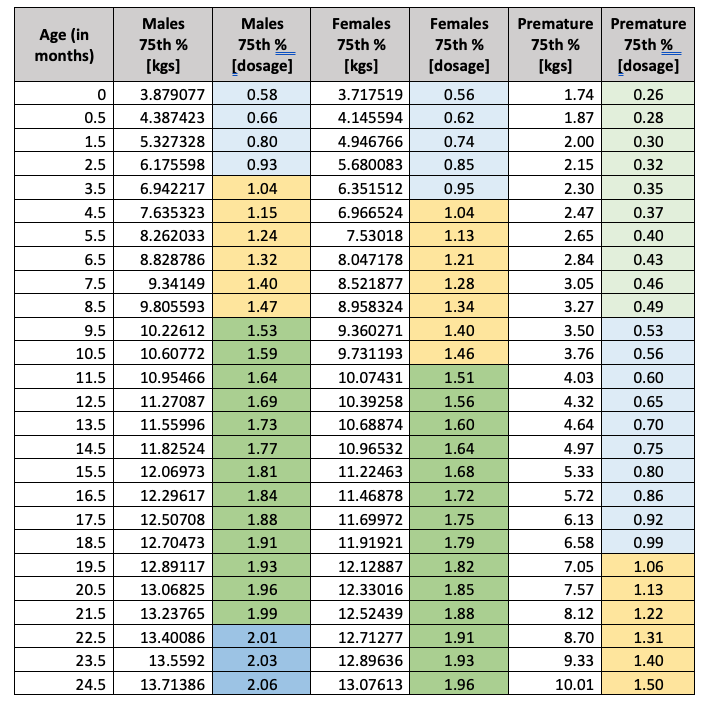
Codoxo calculated the dosage amounts for all populations for the 5th, 25th, 50th, 75th and 95th percentiles and the complete tables are available as an addendum to this article. These tables may be a valuable countermeasure for health plans in the pre-authorization process when the pediatric patient’s history includes the premature birth ICD-10 CM codes for gestation periods less than 37 weeks, low birth weights, or documentation of the pediatric patient’s growth percentile as 5% or less.
Preauthorization assures payment for the approved services or drugs and does allow a health plan to recover the payments in a retrospective review. In our review of our partner’s data, we identified patients with potentially excessive dosages using a premature pediatric patient growth chart. The expectation is that a minimal number of the preauthorization requests would include excessive dosages; however, keep in mind that one extra 50mL vial is approximately $1500 multiplied by five treatments for each patient.
Recommendations
Codoxo suggests using the premature age-to-weight compensatory growth charts as a countermeasure to assess the requested Synagis dosage from the prescribing providers for pediatric patients whose history contains ICD-10 CM codes specific to low birth weights, gestation periods less than and percentile benchmarks.
At the conclusion of the RSV season in your region, we recommend identifying the high-risk pediatric patients whose dosages are 100mL or more, especially those under a year old who were born prematurely and reviewing the patient records that precede the Synagis request. The objective would be to identify an individual prescriber or group or prescribers whose requested dosages appear to exceed the amount needed compared to their expected growth.
Should your health plan adopt the countermeasure prior to the 2023-2024 RSV season, it could be a valuable tool to identify excessive dosage requests, essentially an option for preauthorization, pre-pay review. Moreover, educating the prescribing providers on proper dosage requests promotes collaboration and the opportunity for future savings.
References:
https://www.cdc.gov/rsv/research/rsv-net/dashboard.html
https://www.cdc.gov/rsv/references.html
https://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/
https://synagishcp.com/synagis.pdf
https://synagishcp.com/content/pdfs/SYNAGIS%20Coding%20Resource.pdf
https://www.infantchart.com/olsenweightforage.php
https://www.cdc.gov/nchs/nvss/vsrr/natality-dashboard.htm#
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2821086/
https://www.cdc.gov/growthcharts/html_charts/wtageinf.htm#males
https://www.cdc.gov/growthcharts/html_charts/wtageinf.htm#females