Covid-19 Vaccine Updates and What to Know
There have been many reports of the manufacturers of Covid-19 vaccines submitting additional requests for Emergency Use Authorization (EUA) for children’s dosing and boosters for those fully vaccinated. Codoxo thought it was important to provide a status on what has been approved and the new American Medical Association (AMA) codes that have been released.
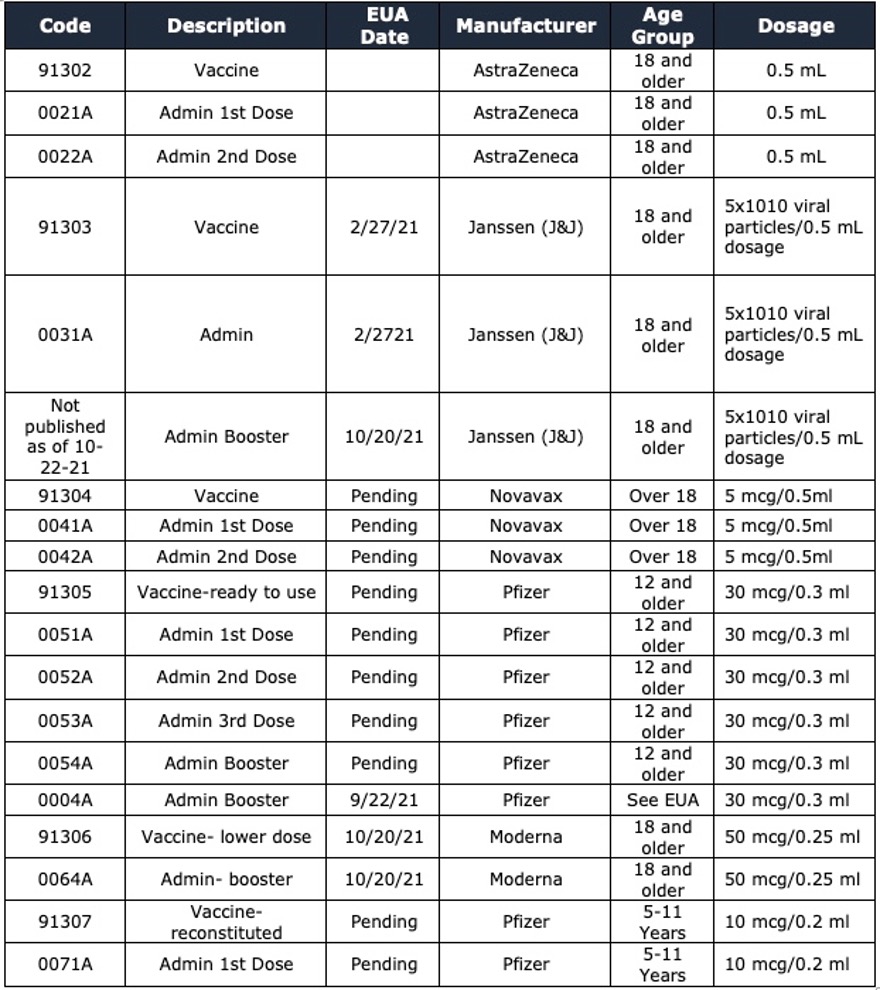
Please note the following:
-
- Some approvals are still pending approval by the Food and Drug Administration (FDA).
- Pfizer is marketing their Covid-19 vaccine under the name of Comirnaty, so do not be confused by the name if you see this in some of the reference materials.
- AstraZeneca and Novavax vaccine codes are included for any foreign claims you may receive since they are not available in the USA.
- Moderna and Janssen received EUA on 10-20-21 for boosters.
- The AMA, as of this writing, has not released a separate code to report the Janssen (J & J) booster, but the dosage is the same for the initial vaccine with a 2-month interval between vaccines and is available for those 18 years of age or older.
- Mixing manufacturers of booster injections is allowed. Please refer to the CDC (Centers for Disease Control) website for the eligibility requirements for each manufacturer.
- On 10-21-21, the CDC has announced changes to their recommendations for those eligible for a booster vaccine.
All information contained in this release is current as of this writing. Please review the AMA, FDA, and CDC websites for further updates to stay abreast of the most current codes and information.
References:
- American Medical Association – https://www.ama-assn.org/practice-management/cpt/covid-19-cpt-vaccine-and-immunization-codes
- Center for Diseases – https://www.cdc.gov/vaccines/programs/iis/COVID-19-related-codes.html
- Food and Drug Administration – https://www.fda.gov/news-events/fda-newsroom
- Food and Drug Administration 9-22-21 EUA Pfizer Booster Guide – https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations
- Food and Drug Administration 10-20-21 EUA for Moderna and Janssen Boosters – https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-additional-actions-use-booster-dose-covid-19-vaccines?utm_medium=email&utm_source=govdelivery
- CDC Press Release on Boosters 10-21-21 – https://www.cdc.gov/media/releases/2021/p1021-covid-booster.html
If you are interested in learning more about Codoxo’s forensic AI platform or would like to speak to Codoxo team member, contact us via info@codoxo.com.
